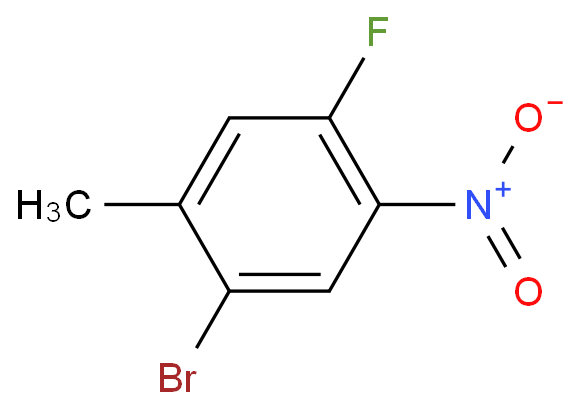
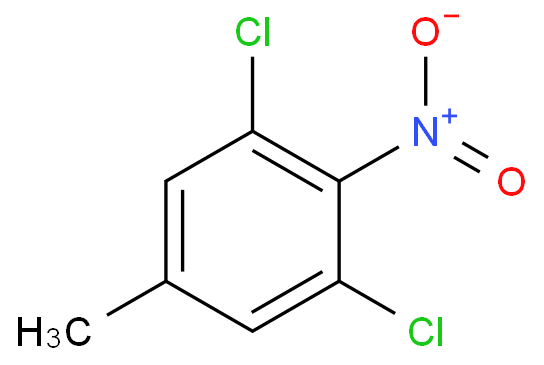
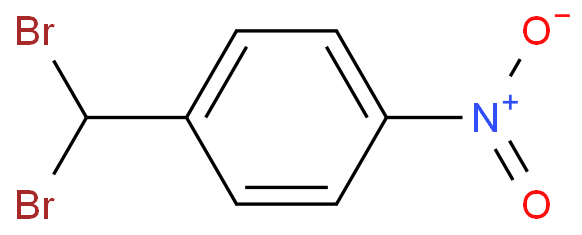
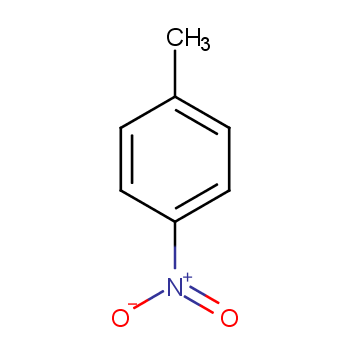
I'd doubt that exclusive nitration of toluene in para position is possible in homogenous solution.
But imagine to stuff a toluene molecule into a very narrow "tube". This tube will block any attack at C-2 (and C-3), while a nitrating agent may approach C-4.
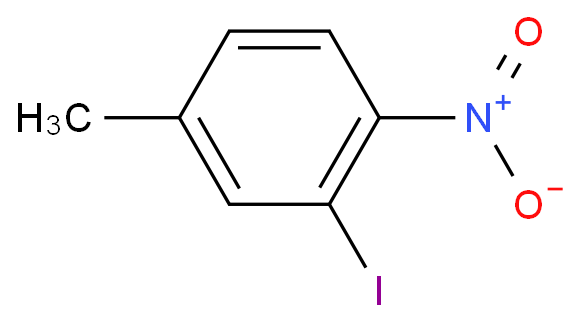
Exactly this has been done in the vapour phase nitration of toluene with nitric acid over a zeolite (ZSM-5). See: Kalbasi, R.; Ghiaci, M.; Massah, A. Highly selective vapor phase nitration of toluene to 4-nitro toluene using modified and unmodified H3PO4/ZSM-5. Applied Catalysis A: General 2009, 353 (1), 1–8. DOI: 10.1016/j.apcata.2008.10.013.
I think It can be done. But it's a rather long process.First prepare nitrobenzene from benzene by nitration.Then, convert it to aniline by adding a reducing agent(preferably Sn/Hcl).Then, Convert the NH2 group to NHCOCH3 by adding ethanoic anhydride in acetic acid(Which is a weaker activating group than NH2 but a stronger one as compared to CH3).Then, Add the methyl group by friedel-crafts alkylation. Then, convert NHCOCH3 back to NH2 by hydrolysis.Then, convert aniline to diazonium chloride by adding NaNo2 in Hcl. Finally convert diazonium chloride to Nitro group by Adding NaNo2/Cu. U get p-Nitrotoluene. But, I am not too sure this is acceptable.