 |
Brexpiprazole is the first compound developed jointly by Otsuka Pharmaceutical and Lundbeck that acts as a dopamine partial agonist, a partial agonist of 5-HT1A receptors, and an antagonist of 5-HT2A receptors. It was launched in the United States on July 10, 2015, for the treatment of adult schizophrenia and as an adjunctive therapy with antidepressants for the treatment of severe depression in adults. 7-Hydroxyquinolinone is an important intermediate in the synthesis of brexpiprazole.
According to publicly available literature [1], 7-Hydroxyquinolinone is prepared by using acetone and ethyl acetoacetate as raw materials to form 3-(2,4-dioxo-cyclohexyl)-ethyl acetoacetate, followed by protection, amide exchange, and cyclization to obtain 7-Hydroxyquinolinone.
A method for preparing 7-Hydroxyquinolinone is disclosed in this invention, which involves adding 7-hydroxy-3,4-dihydro-2(1H)-quinolinone raw material to an inorganic base solution, heating to the reaction temperature of 40-80°C, oxidizing with activated manganese dioxide, and separating 7-hydroxyquinolinone from the reaction mixture to obtain the final product. This method has the advantages of a short reaction route, simple process, readily available raw materials, a product yield of over 60%, and a 20-30% reduction in production costs, making it suitable for industrial applications.
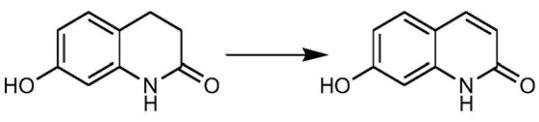
Figure 1 Synthesis Reaction of 7-Hydroxyquinolinone
In a 500mL three-neck flask, add 240mL water, 36.8g sodium hydroxide, stir until completely dissolved, then add 30g 7-hydroxy-3,4-dihydro-2(1H)-quinolinone, stir well; heat to 50-60°C, then add activated manganese dioxide 96g in portions (8-10g every 5 minutes), maintain the reaction temperature at 50-60°C for 7-8 hours; identify the reaction endpoint with TLC (eluent: dichloromethane-methanol, volume ratio 20:1), cool the system to 30-40°C after the reaction, filter, wash the filter cake with 50mL 8% sodium hydroxide solution; transfer the filtrate to a three-neck flask, add 3g activated carbon, heat to 60-70°C, stir for 1-2 hours for decolorization; filter the decolorized solution, adjust the pH to 2-3 with concentrated hydrochloric acid, filter again, obtain the product filter cake, wash the filter cake with 50mL water, dry the filter cake at 40-50°C to obtain 7-Hydroxyquinolinone 18.0g, with a yield of 61%.
[1] CN106749007B - A method for preparing 7-Hydroxyquinolinone
 |