 |
BICALUTAMIDE is a drug that is widely used in the treatment of prostate cancer. The mechanism of action is essential to understand its therapeutic effect. Before using the drug, please consult a doctor to ensure that it is suitable for you.
Introduction:
1. BICALUTAMIDE mechanism
BICALUTAMIDE is a new type of non -steroidal antibodium preparation. The mechanism of action is to combine withrogen receptor in the prostate level to form a complex withrogen receptor, blocking DHT and the nucleus receiving The combination of body makes DHT cannot play a role, thereby suppressing the growth of prostate cell carcinoma that inhibit the growth of male hormones. It can also inhibit testosterone transition into DHT. DHT is the activity of testosterone in the cells, so therogens are used to prostate The growth stimulus effect is suppressed to a large extent. It is manifested in the synthesis of DNA significantly inhibitory, resulting in atrophy of prostate cancer and reaching the role of anti -tumor (see Figure).
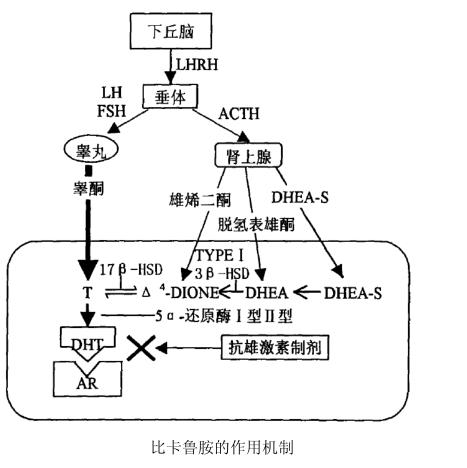
BICALUTAMIDE is an external rotor in the commodity Constance. The results of the experiments in the body show that R-BICALUTAMIDE's antibodium activity is 60 times that of its S-type isomer, the BICALUTAMIDE of BICALUTAMIDE The specificity is particularly strong, has good tolerance, is convenient for administration, and the patient will not have any bodies activity effects after taking it [10], and may have adverse reactions such as flushing and weakness.
2. Pharmacy research
( 1) Anti -roged androgen effects
BICALUTAMIDE can be combined with the prostate androgen hormone receptors of monkeys, dogs, rats, people, etc. to inhibit gene expression androgen to promote cell growth. Its role is peripheral selectivity. BICALUTAMIDE is an external anti-rotor. It basically only appears in the action of anti-androgens in the (R)-type structure, and the (S)-type structure is basically not.
( 2) Impact on animal testicles
The effect of BICALUTAMIDE can cause dog's testicles and prostate atrophy. Oral ED 50 is 0.1 mg/kg, and its role is about 50 times the strength of fluorheide.
( 3) The effect of hormone -dependent cancer model
In the rats hormone -dependent cancer model, after half a month with BICALUTAMIDE treatment, the weight of the semen and prostate is significantly reduced, and the concentration of serum testosterone has increased. BICALUTAMIDE significant inhibitory LNCAP/FGC prostate cancer cell growth is still active even under the condition of removing hormones. However, when the testicular propionate is added, this inhibitory effect will be eliminated, and its mechanism is mainly mediated byrogen receptor.
3. Pharmacokinetics research
BICALUTAMIDE is an external anti-rotor, and its anti-androgen activity almost exists completely (R) -The performers, and (s) -The activity in the body (if there are, if there are any If you)). (R) -BICALUTAMIDE is slow and saturated, but absorption is not affected by food. Its plasma is eliminated for a long period (1 week), and it accumulates about 10 times in the plasma daily. (S) -BICALUTAMIDE absorption and clearance is faster; (R)-BICALUTAMIDE's steady-state concentration (CSS) is 100 times higher than (S) -BICALUTAMIDE. With the linearity of the dose, CSS can reach 50 mg, but at a higher dose, it shows non -linearity increase and reaches a stable state above 300 mg. The Japanese CSS is higher than that of white people, but has nothing to do with the incompleteness, weight, or age of renal function. Although mild to moderate liver function will not affect pharmacokinetics, there is evidence that (R) -BICALUTAMIDE is eliminated slower than patients with severe liver dysfunction. The BiCalutamide metabolites are excreted by urine and feces almost equally. There are very few drugs excreted in urine or no primary drugs; on the contrary, the primary drugs are mainly used in plasma. The BiCalutamide in the feces is considered a hydrolyzed and undesurized drugs from the aldosinoside of the BICALUTAMIDE. BiCalutamide seems to be almost fully cleared through metabolism; for (R) -BICALUTAMIDE, this is mainly mediated by cytochrome P450 (CYP), but glucosalization is (S) the main metabolic pathway of (S) -Bicalutamide. (S) -Bicalutamide is metabolized by CYP3A4 in vitro, and this kind of homogenase is likely to be responsible for (R) -Bicalutamide's metabolism. In vitro data shows that (R) -BICALUTAMIDE may inhibit CYP3A4 and inhibit CYP2C9, 2C19 and 2D6 to a small extent.
4. Advantages
BICALUTAMIDE has many advantages compared to traditional antiaroxic drugs. Compared with the active metabolites of fluorotide —The hydroxyl fluorotamine, the affinity of BiCalutamide and prostate androgen receptor is 4 times higher, and the affinity of pituitary androgen receptor is 10 Double, its high affinity for androgen receptors is the reason why its anti -tumor activity is stronger than fluorheetamine. The half -life of BiCalutamide is longer, so it only needs to be administered once a day, and fluorheetamine needs to be taken three times a day. There are significant differences in structure and composition. BICALUTAMIDE can inhibit therogen receptor that mutates fluorotamine stimulation. Therefore, it can still achieve good results using BiCalutamide to resist patients with fluidide. The drug has strong specificity, simple administration, good tolerance, and relatively small side effects. Occasionally, adverse reactions such as flushing or weakness may occur.
refer to:
[1] Teng Teng. BICALUTAMIDE Quality Standard Evaluation and Research [D]. Zhejiang University, 2017.
[2] Wang Jianzhong. BICALUTAMIDE Research on hand -splitting and pharmaceutical dynamics of the heterogeneity [D]. Shanghai Jiaotong University, 2011.
[4] Li Yunlong. The synthesis and process improvement of BICALUTAMIDE [D]. Wuhan University of Engineering, 2017.
[5] Xie Yuan. BICALUTAMIDE intermediate 4-amino-2-trifluorophyl methyl phenylphenylene synthesis [D]. Dalian University of Technology, 2013.
[6] Cockshott I D. BiCalutamide: Clinical Pharmacokinetics and Metabolism [J]. Clinical Pharmacokinetics, 2004, 43: 855-87888888888888888888888888.
[7] https://www.sciencedirect.com/topics/biochemistry-tenetics-nd-molecular-biology/bicalutamide
 |