 |
5,6-DIHYDROURACIL is an intermediate product of uracil decomposition metabolism, which can form dihydrouridine with ribose through a C-N glycosidic bond. In RNA, it can form complementary pairs with adenine. 5,6-DIHYDROURACIL is a compound. Here is a detailed introduction to the properties, uses, synthesis, and safety information of 5,6-DIHYDROURACIL.
5,6-DIHYDROURACIL is a colorless crystalline powder. It is insoluble in water but has good solubility in some organic solvents.
5,6-DIHYDROURACIL can be used to synthesize DNA polymerase inhibitors analogs. Additionally, it is a metabolite of uracil and can serve as a marker for identifying dihydropyrimidine dehydrogenase (DPD) deficiency.
(1) 56.0g (0.40mol) of acrylic acid and 120mL of chloroform are added to a 250mL four-necked reaction flask, mixed well under mechanical stirring, then the flask is cooled to 0°C. Hydrogen bromide gas (38.9g, 0.48mol) is slowly introduced under flow control, and the reaction continues for 1h after the gas is complete. The reaction is then allowed to continue at room temperature for 12h, and after solvent evaporation by rotary evaporation, 3-bromopropanoic acid is obtained.
(2) Under nitrogen protection, 88.4g (0.4mol) of 3-bromopropanoic acid is slowly added to a reaction vessel, followed by 300mL (2.16mol) of ammonia solution. The reaction system is sealed, heated to 80°C, and maintained at 80°C for 2h. After the reaction, the material is released, cooled, filtered, and dried to obtain 3-aminopropanoic acid.
(3) 62.0g (0.4mol) of 3-aminopropanoic acid and 80.3g (0.44mol) of 20% concentrated hydrochloric acid are added to a 500mL three-necked reaction flask. The flask is heated to 100°C, and then 195g (0.6mol) of 20% concentrated sodium hydroxide solution is added dropwise within 1h. After the dropwise addition, the reaction is maintained at 100°C for 3h, then cooled, crystallized, filtered, and dried to obtain crude 5,6-DIHYDROURACIL. Finally, recrystallization with 184g (4.0mol) of anhydrous ethanol yields white 5,6-DIHYDROURACIL crystals.
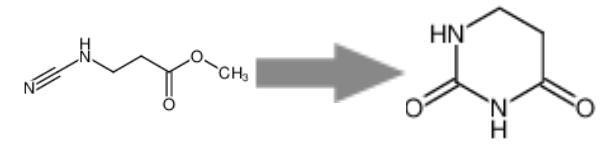
Figure 1 Synthesis Reaction of 5,6-DIHYDROURACIL
5,6-DIHYDROURACIL is generally considered a low-toxic substance, but appropriate safety measures should still be taken.
Avoid skin, eye, and respiratory contact during operation. In case of accidental contact, rinse with plenty of water immediately.
When storing, keep it in a dry, cool place, away from oxidants and sources of ignition.
When using 5,6-DIHYDROURACIL, follow safety procedures and wear appropriate protective equipment, such as laboratory gloves and safety goggles.
[1] CN106831606A - A method for preparing 5-trifluoromethyl-5,6-DIHYDROURACIL
 |