 |
Platinum is an important member of the platinum group metals and is widely used in automotive, electronics, batteries, pharmaceuticals, petrochemicals, and other fields. Hexachloroplatinic acid, as one of the important platinum-based compounds, can be used to prepare catalysts, chemical electrodes, analytical reagents, and more.
The preparation of hexachloroplatinic acid from platinum mainly involves chemical dissolution and electrochemical dissolution. The chemical dissolution method primarily uses hydrochloric acid as a solvent and adds an oxidant. Common oxidants include nitric acid, H2O2, Cl2, etc. Among them, aqua regia formed by hydrochloric acid and nitric acid in a volume ratio of 3:1 is the most widely used. Under heating conditions, aqua regia reacts with platinum to generate hexachloroplatinic acid. After platinum is dissolved, hydrochloric acid is added to the solution in batches to remove nitrate ions. The preparation of hexachloroplatinic acid by aqua regia dissolution has the characteristics of strong operability and fast dissolution rate. However, it consumes a large amount of hydrochloric acid and nitric acid, and emits a large amount of NOX, causing environmental pollution. The electrochemical dissolution method involves dissolving platinum by electrolyte to make the platinum anode undergo a dissolution reaction. The dissolution effect of platinum is mainly related to factors such as current density, electrolyte, and time. The electrochemical dissolution method for dissolving platinum to prepare hexachloroplatinic acid has the characteristics of stable reaction, high safety, and low acid consumption. However, it also has problems such as slow dissolution rate and incomplete dissolution.
The process of AC electrochemical dissolution of platinum in industrial tests is shown in the figure below. First, concentrated hydrochloric acid and a small amount of nitric acid are added to the electrolytic cell made of polytetrafluoroethylene. Then, a uniformly thick platinum sheet is vertically inserted into the electrolytic cell. The AC power supply is connected to the graphite electrode covered with an insulating layer from the periphery through a contact regulator to carry out electrochemical dissolution. The gas generated during the dissolution process is condensed by a condenser and then pumped out by a water-ring vacuum pump.
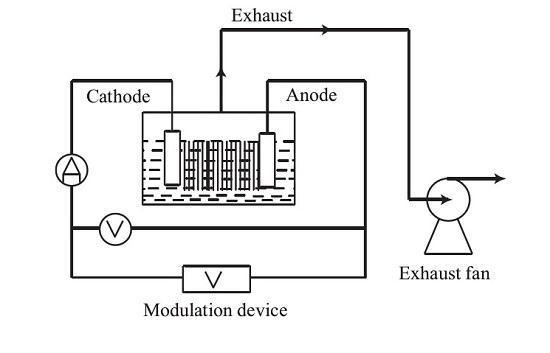
The principle of AC platinum dissolution: Platinum to be dissolved serves as the electrode, and hydrochloric acid serves as the electrolyte solution. When the current passes through the electrolyte, platinum on the metal anode undergoes a dissolution reaction to generate Pt4+. Pt4+ forms a complex ion with the anion Cl- in the solution, which does not precipitate on the cathode, and the formed complex ion then forms H2PtCl6 with the H+ in the solution.
Weigh the platinum block, roll it into a uniformly thick platinum sheet, then cut it into pieces, and wash it with distilled water and anhydrous ethanol. Insert the cleaned platinum sheet into the grid and place the grid in the electrolytic cell. Add a suitable amount of deionized water, hydrochloric acid, and nitric acid to the electrolytic cell. Turn on the current, adjust the regulator to the set voltage and current, and start electrolysis. The exhaust gas generated during the electrolysis process is pumped out by a vacuum pump. After a certain period of electrolysis, stop and transfer the electrolyte in the electrolytic cell to a storage tank. Rinse the electrolytic cell with deionized water, and the rinsing solution is also transferred to the storage tank. Weigh the platinum sheet that has not been completely dissolved.
1) The optimal conditions for industrial AC electrochemical dissolution of platinum to prepare hexachloroplatinic acid are: current density of 355 mA/cm2, hydrochloric acid concentration of 7-8 mol/L, nitric acid concentration of 1.5 mol/L, and dissolution time of 15.5 hours.
2) Precipitation method is used to analyze the platinum content in the hexachloroplatinic acid solution. The deviation of the analysis result compared with the standard hexachloroplatinic acid solution is less than 0.04%, which can be used for rapid analysis of platinum content in hexachloroplatinic acid.
3) The hexachloroplatinic acid prepared by electrochemical dissolution using naphtha as the raw material for the continuous reforming catalyst is comparable to the standard catalyst in catalytic performance.
4) Compared with aqua regia dissolution, electrochemical dissolution of platinum to prepare hexachloroplatinic acid reduces the consumption of hydrochloric acid and nitric acid, and reduces the amount of exhaust gas, resulting in good economic and social benefits.
 |