 |
Phenyl alkyl ethers are commonly prepared by the cleavage of ether bonds to remove alkyl groups, which is a common organic reaction. The cleavage of ether bonds can be achieved through the action of Bronsted acids or Lewis acids. Common Bronsted acids include HCl, HBr, and HI, while common Lewis acids include BBr3 and AlCl3. However, when the substrate contains acid- or base-sensitive functional groups, it can be challenging to remove the methyl group using these methods. For example, eugenol or 2-Allylphenol can only be obtained with moderate to low yields using these methods.
In order to address this issue, Lange developed a method using AlCl3-tertiary amines, which was successfully applied to the demethylation reaction of hydroxybenzyl ethers such as vanillin (US3256336). However, the reactivity of AlCl3 in breaking ether bonds is generally low, resulting in low yields when used for substrates containing acid-sensitive functional groups like eugenol. To overcome this, Arifin et al. developed the AlCl3-DMS method (Indon.J.Chem.2015,15,77), but the yield for the demethylation reaction of eugenol was still very low (around 30%). CN106278825A discloses a method using AlCl3-pyridine to break ether bonds, which successfully removes the methyl group from eugenol in nearly quantitative yield. However, the method can only be used for ortho-hydroxyphenyl alkyl ethers due to the complexation of pyridine with diiodoaluminum, and it cannot be applied to phenyl alkyl ethers without ortho-hydroxy groups. 2-Allylphenol serves as an excellent example of application in this preparation.
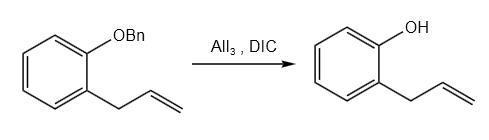
Synthesis 1: To a 100 ml pear-shaped flask, add 1.538 g of aluminum triiodide, 40 ml of acetonitrile, 0.042 g of DIC, and 0.761 g of 2-allylphenyl benzyl ether. Heat the mixture to 80°C and stir for 18 hours. Stop stirring and cool the flask to room temperature. Add 10 ml of 2 mol/L dilute hydrochloric acid to the flask and acidify. Extract with 50 ml portions of ethyl acetate three times. Combine the organic phases and wash with 10 ml of saturated sodium hydrogen sulfite solution, followed by 10 ml of saturated saline solution. Dry over anhydrous magnesium sulfate, filter, and evaporate the filtrate using a rotary evaporator. Purify the residue by rapid column chromatography (using ethyl acetate/petroleum ether = 1:4, volume ratio) to obtain 0.314 g of 2-allylphenol (pale yellow oily liquid, yield: 68%).
Synthesis 2: To a 100 ml pear-shaped flask, add 2.144 g of aluminum triiodide, 40 ml of acetonitrile, 0.149 g of DIC, and 1.445 g of 2-allylphenyl dodecyl ether. Heat the mixture to 80°C and stir for 18 hours. Stop stirring and cool the flask to room temperature. Add 10 ml of 2 mol/L dilute hydrochloric acid to the flask and acidify. Extract with 50 ml portions of ethyl acetate three times. Combine the organic phases and wash with 10 ml of saturated sodium hydrogen sulfite solution, followed by 10 ml of saturated saline solution. Dry over anhydrous magnesium sulfate, filter, and evaporate the filtrate using a rotary evaporator. Purify the residue by rapid column chromatography (using ethyl acetate/petroleum ether = 1:4, volume ratio) to obtain 0.201 g of 2-allylphenol (pale yellow oily liquid, yield: 31%).
[1] Jingchu University of Technology. A method for breaking ether bonds in phenyl alkyl ethers: CN201710682010.1[P]. 2020-09-22.
 |