 |
 |
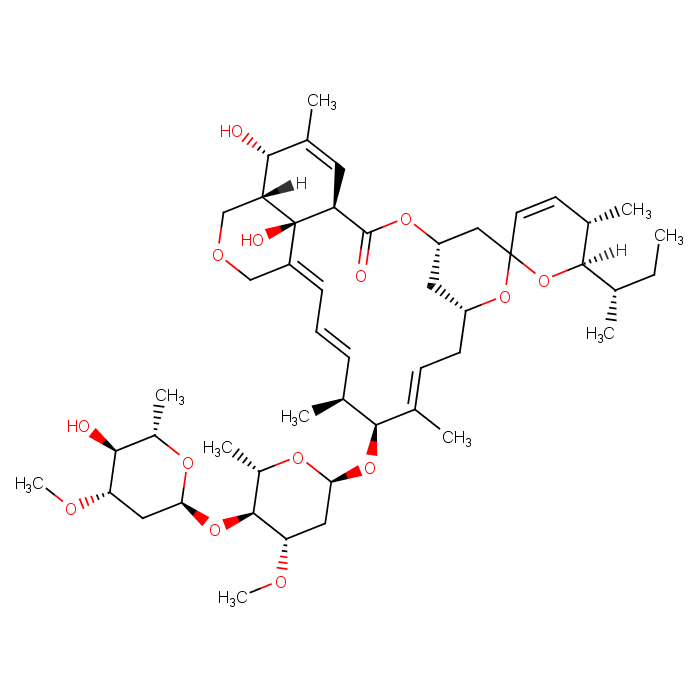
Abamectin(C49H74O14) is an organic molecule. Abamectin is a macrocyclic lactone derived from the bacterium Streptomyces avermitilis. It is commonly used as a pesticide for the control of a variety of agricultural pests, including mites, insects, and worms. Abamectin acts by interfering with the nervous system of pests, causing paralysis and ultimately death.
Abamectin is a potent compound that is effective against a broad spectrum of pests. It is primarily used in both agricultural and veterinary settings. In agriculture, it is used to protect crops from harmful pests, while in veterinary medicine, it is used to treat parasitic infections in livestock and pets, such as heartworm and intestinal worms.
Abamectin is typically stored in a cool, dry place away from direct sunlight to preserve its effectiveness. It is important to follow safety guidelines when handling Abamectin, as it can be toxic to non-target organisms such as fish and bees. Proper protective equipment should be worn to avoid exposure.
View more+(1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2R)-2-Butanyl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.14,8. (2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-2-Butanyl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.14,8.020 ,24]pentacosa[10,14,16,22]tetraen]-12'-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside 020,24]pentacosa[10,14,16,22]tetraen]-12'-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-β-L-lyxo-hexopyranoside Abamectin 100mg [71751-41-2] AbaMectin (AverMectin B1)(FDA) Abamectin soL Abamectin Solution in Acetonitrile Abamectine 10 ?g/mL in Acetonitrile Abamectine 100 ?g/mL in Acetonitrile AVERMECTIN B1 Avermectin B1a AverMectin B1a-AverMectin B1b Mixt. Avermectin B1b MFCD01769550 Mixture of abamectin B1a and B1b Purity of AbaMectin
Acute toxicity - Oral, Category 2
Acute toxicity - Inhalation, Category 1
Specific target organ toxicity \u2013 repeated exposure, Category 1
Hazardous to the aquatic environment, short-term (Acute) - Category Acute 1
Hazardous to the aquatic environment, long-term (Chronic) - Category Chronic 1
Reproductive toxicity, Category 2
| Pictogram(s) |    |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H300 Fatal if swallowed H330 Fatal if inhaled H372 Causes damage to organs through prolonged or repeated exposure H410 Very toxic to aquatic life with long lasting effects H361d |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P271 Use only outdoors or in a well-ventilated area. P284 [In case of inadequate ventilation] wear respiratory protection. P273 Avoid release to the environment. P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. P280 Wear protective gloves/protective clothing/eye protection/face protection. |
| Response | P301+P310 IF SWALLOWED: Immediately call a POISON CENTER/doctor/\u2026 P321 Specific treatment (see ... on this label). P330 Rinse mouth. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P310 Immediately call a POISON CENTER/doctor/\u2026 P320 Specific treatment is urgent (see ... on this label). P314 Get medical advice/attention if you feel unwell. P391 Collect spillage. P308+P313 IF exposed or concerned: Get medical advice/ attention. |
| Storage | P405 Store locked up. P403+P233 Store in a well-ventilated place. Keep container tightly closed. |
| Disposal | P501 Dispose of contents/container to ... |
none