 |
Introduction:
Thiourea is a compound with important chemical significance that plays an important role in the chemical field. Thiourea is an organic compound containing sulfur and nitrogen, which has a unique structure and properties. In chemical reactions, Thiourea is often used as a reducing agent, complex or catalyst, playing a key role. In addition to the application in chemical synthesis, Thiourea is also widely used in the fields of metal corrosion suppression, drug synthesis, and dye industry. This article will explore the significance and applications of Thiourea in chemistry, and in -depth analysis of its mechanism and value in different fields. By comprehensively understanding the diversity of Thiourea, we can better use the characteristics of this compound to promote the development and innovation of the chemical field.
1. What is Thiourea?
Thiourea CAS is 62-56-6. Organic sulfur compounds are composed of carbon, nitrogen, hydrogen and sulfur atoms. Thiourea chemical formula is SC (NH2) 2. As its name and composition indicate, Thiourea is very similar to urea. In Thiourea, the oxygen atoms of urea are replaced by sulfur atoms. It should be noted that urea and Thiouurea are similar in structure, but it is very different in physical and chemical properties. Thiourea is an organic molecule similar to urea, but contains sulfur instead of oxygen; its chemical formula is CS (NH2) 2, and urea is CO (NH2) 2. Like urea, it is made by inducing substances with similar chemical properties, such as heating of ammonium trails (NH4SCN). Adding hydrogen sulfide to cyanide is a more commonly used production technology. Thiourea contains many chemical characteristics as urea, although it is not widely used. A small amount of Thiourea is mainly used for photography, as a fixed film, producing heat solid resin, as pesticides, textile treatment, and the initial component of certain colors and medicines. At 182 ° C (360 ° F), Thiourea crystals are colorless crystals. It is toxic, although the damage dose is not determined.
It is a bitter white water -soluble crystalline chemical, which can form additional compounds with metal ions to take photos of shadow, rubber vulcanization and synthetic resin production. Thiourea is used to make flame retardant resin and vulcanization promoters. Thiourea is used as a nitrogen paper (sensing copying paper) and almost all other types of copies of paper. This is also used to color the printing printed by Yinmingjiao.
2. The nature of thiourea
( 1) Chemical information
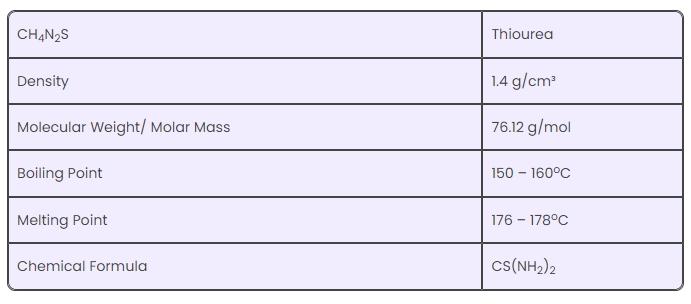
( 2) Physical properties
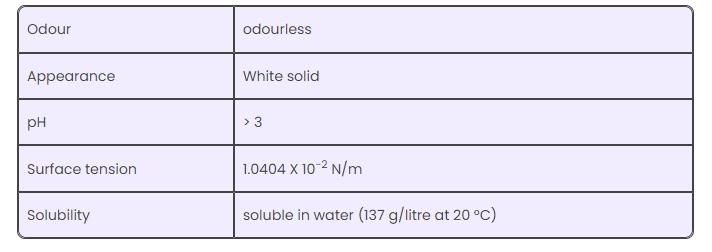
Thiourea is acidic or alkaline? Thiourea pH value > 3 is a neutral compound. It is not acidic or alkaline in pure state. Thiourea solubility: soluble in water (137 grams/liter, 20 ° C). In the aquiaorea, Thiourea can hydrolyze the Thiourea derivative and release hydrogen ions (H+) or hydroxide ion (OH-) according to conditions. In this sense, it can show acid or alkaline behavior based on the pH value of the solution. Thiourea's aminel (-NH2) can accept or provide protons to make it a two-nature nature, which means that it can act as acid and alkali in different cases.
( 3) Chemical properties
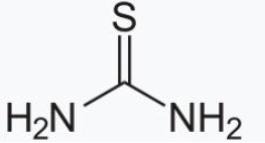
Thiourea structure is as follows:
THIOUREA react with alkyl halide to generate different THIOUREA salt, which is further hydrolyzed to produce sulfenol and urea.
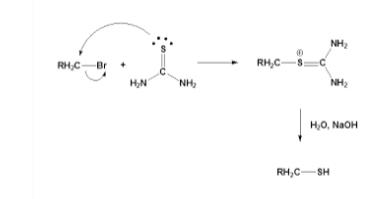
3. Thiourea's chemical behavior
( 1) The response nature of Thiourea in different environments
Thiourea is a compound of chemical formula (NH2) 2CS, which has a variety of reaction properties in various environments. Under acidic conditions, the quality of the Thiourea cation in Thiourea has enhanced its reaction activity in the nuclear replacement reaction. In an alkaline solution, Thiourea is generated by generating Thiouorea, changing its chemical behavior of parenting substances. This behavior that depends on the PH value makes Thiourea a valuable reagent in synthetic organic chemistry, and its reaction activity can be adjusted according to specific reactions.
( 2) interaction of Thiourea and other compounds
In the metal mathematical, Thiourea is used as a two -toothed ligand and is equipped with sulfur atoms and nitrogen atoms. This chelating ability plays a key role in the synthesis of matches and catalysts. Thiourea and metal ions show unique interaction, forming a stable mathematical with silver, copper and mercury.
4. The application of thiourea
(
In agriculture, Thiourea is an important tool for improving crop yield and promoting plant growth. It stimulates the ability of plant nutrition absorption to make it a popular additive in fertilizer and soil conditioning agent. By promoting the absorption of essential nutrients such as nitrogen and phosphorus, Thiourea supports the strong development of plants, which ultimately leads to the improvement of crop yield. In addition, Thiouorea's insecticidal characteristics make it a effective agent to protect crops from various diseases and insect pests, which helps sustainable agriculture.
( 2) The application of Thioorea in photography
In the field of photography, Thiourea plays a vital role in image production, especially in the development of photography. As a key component of fixed liquid, Thiourea helps remove the unre exposed silver crystals from the photographic emulsion, thereby stabilizing the image image. This process prevents photos that do not want fading or discoloration over time to ensure their life and the quality of files. In addition, the use of Thiourea processing technology to protect image integrity during the washing process to help photographers get the best results and maintain the visual integrity of their works.
( 3) The application of Thiourea in the textile industry
In the textile industry, Thiourea completely changed the fabric dyeing process, providing innovative solutions for the realization of the required color tone and improvement of fabric printing technology. Its unique chemical characteristics can accurately control dye penetration and color fastness, thereby generating vibrant and durable tones on textile materials. The role of Thiorea in fabric dyeing is not only coloring; it also helps improve the adhesion of the dye to the surface of the fabric, thereby improving the entire printing process. This not only improves the beauty of textiles, but also ensures excellent durability and fading, which meets the strict quality standards of the textile market.
5. Is Thiourea toxic?
Thiourea looks like a white crystal, which is flammable. When it is exposed in the fire, it will emit a unpleasant or toxic smell. Contact Thiourea will have a negative impact on health and may cause poisoning. It enters the human body by inhaling its gas solution and swallowing. It is known that when it is repeated or long -term contact with Thiourea, it will cause skin sensitivity and various thyroid health problems. Studies have shown that high -concentration Thiourea may lead to adverse effects, such as stimulating the respiratory tract and skin, and potential mutation and carcinogenic characteristics in some cases. Therefore, strict safety measures must be taken when dealing with Thiourea, including personal protection equipment such as gloves, goggles and respirators to minimize contact. Proper ventilation and control schemes should be formulated to prevent inhalation or intake of Thiourea. In addition, regular risk assessment and monitoring contact levels are essential for ensuring the safety of using Thiourea's personal security in the industry, agriculture or laboratory environment.
6. Thiourea MSDS
Material Security Data Table ( MSDS), also known as security data table (SDS), is used to provide detailed information about chemicals, including its security, danger and emergency processing information. These forms are usually provided by chemical manufacturers, suppliers or distributors, and aims to help users and staff understand how to safely handle, store, transport, and dispose of chemicals. Research reports Thiorea msds are:
(
(
( 3) The safety first aid measures of Thiourea include: skin contact: immediately wash the skin with soap and water. Eye contact: Immediately rinse the eyes with water for at least 15 minutes and seek medical treatment. Inhale: Immediately move the patient to the fresh air. If you have difficulty breathing, you should seek medical treatment immediately. Eat: immediately seek medical treatment and inform the doctor the substance ingredients.
( 4) The storage and use of Thiourea should be paid attention to, and should be stored in dry, ventilated, cool places, avoid direct sunlight and high temperature, wear personal protection equipment, control the amount of usage, and properly handle it properly Waste.
( 5) The use and storage of Thiourea need to comply with relevant laws and regulations and security regulations to ensure safety and environmental protection.
7. Conclusion
Thiourea is a compound with extensive use and potential. From the role of increasing crop yield and prevention of insect pests in agriculture, to its contribution to the process of promoting image production and fabric dyeing in the photography and textile industry, Thiourea shows extraordinary adaptability. Although there is concerns about its toxicity, cautiously handling and obeying the security agreement can reduce the risks related to use. Looking forward to the future, further exploring the nature and application of Thiourea is expected to continue to innovate and progress in various fields. Through in -depth research and discovery of new uses, researchers can use the entire potential of Thiourea to cope with challenges and promote progress in the fields of agriculture, photography, and textiles.
refer to:
[1] https://en.wikipedia.org/wiki/thiourea
[2] https://byjus.com/chemistry/thiourea/
[3] https://www.vedantu.com/chemistry/thiourea
 |