 |
5-Bromo-2-methylbenzoic acid, also known as 5-Bromo-2-methylbenzoic acid, is a grayish-white solid at room temperature and pressure. It is commonly used as an organic synthesis and pharmaceutical chemistry intermediate, with various applications in the preparation of drug molecules and bioactive molecules, as well as fundamental chemical research. For example, 5-Bromo-2-methylbenzoic acid can be used in the synthesis of the blood glucose-lowering drug molecule canagliflozin.
5-Bromo-2-methylbenzoic acid can be used in the synthesis of the blood glucose-lowering drug molecule canagliflozin. Canagliflozin belongs to the class of sodium-glucose co-transporter 2 inhibitors, also known as SGLT2 inhibitors. It is a novel oral antidiabetic drug that reduces the reabsorption of filtered glucose, increases urinary glucose excretion, and delays the generation and absorption of glucose during digestion, thereby controlling blood glucose levels in patients with type 2 diabetes. Recent clinical studies have also shown that canagliflozin can reduce the risk of hospitalization for heart failure and kidney failure.
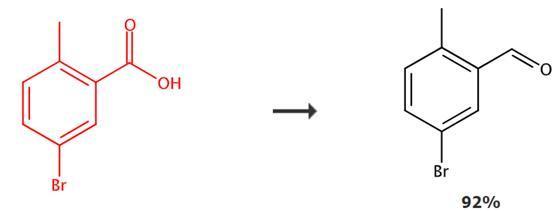
Figure 1: Application transformation of 5-Bromo-2-methylbenzoic acid
At 0°C, a solution of 5-Bromo-2-methylbenzoic acid (91g, 425 mmol) in tetrahydrofuran (500 mL) is gradually heated to room temperature while stirring, after which the reaction mixture is cooled back to 0°C and quenched with methanol and water. The organic layer is extracted with ethyl acetate and separated, followed by washing the organic layer with saturated saltwater. The organic phase is dried over anhydrous MgSO4, filtered, and the solvent is evaporated under vacuum to obtain benzyl alcohol as an intermediate. Dess-Martin oxidant (216g, 510 mmol) is added to a solution of the above benzyl alcohol in dichloromethane (400 mL) at 0°C, and the reaction mixture is stirred at room temperature for 2 hours. After the reaction, the mixture is cooled to 0°C and slowly quenched with saturated NaHCO3 solution, stirring the reaction mixture until no gas is produced. The organic layer is separated and washed with saturated saltwater twice, followed by drying the organic layer over Na2SO4 and filtration. The solvent is evaporated under vacuum. The crude residue is purified by silica gel column chromatography to obtain 5-bromo-2-methylbenzaldehyde.
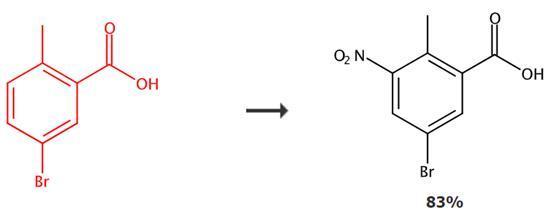
Figure 2: Application transformation of 5-Bromo-2-methylbenzoic acid
At -10°C, a mixture of 5-Bromo-2-methylbenzoic acid (20g, 93 mmol) and concentrated H2SO4 (100 mL) is stirred for 10 minutes, followed by slow addition of a mixture of KNO3 (9.39g, 93.023 mmol) and concentrated H2SO4 (100 mL) to the reaction system. The resulting mixture is stirred at -10°C for 1 hour. After the reaction, the reaction mixture is poured into ice-cold water, resulting in the precipitation of a solid. The precipitate is filtered and dried under vacuum to obtain 5-bromo-2-methyl-3-nitrobenzoic acid.
[1] Ding, Yuyang et al Bioorganic & Medicinal Chemistry Letters, 25(14), 2744-2748; 2015
[2] Sawant, Ajay S. et al Medicinal Chemistry Research, 29(1), 17-32; 2020
 |