
|
Sodium bisulfite, with the chemical formula NaHSO3, has the CAS number 7631-90-5. It is a white crystalline powder with a sulfurous odor. Sodium bisulfite is an inorganic compound that consists of one sodium cation (Na+) and one bisulfite anion (HSO3-). This compound is highly soluble in water. It is important to handle sodium bisulfite with caution as it can cause skin and eye irritation. Ingestion or inhalation of sodium bisulfite may cause respiratory and gastrointestinal irritation. It is also important to avoid exposure to high temperatures or open flames, as sodium bisulfite may release toxic fumes. Sodium bisulfite is primarily used as a food preservative and antioxidant. It is also used in the production of paper and pulp, as a reducing agent in various chemical processes, and in water treatment to remove chlorine and prevent oxidation.
Applicable Fields
Food Preservation: Sodium bisulfite is commonly used as a food preservative due to its ability to inhibit the growth of bacteria and other microorganisms. It acts as an antioxidant, preventing the oxidation of food and extending its shelf life.
Paper and Pulp Industry: Sodium bisulfite is used in the production of paper and pulp. It helps to break down lignin, a complex polymer found in wood, and improves the brightness and quality of the paper.
Chemical Processes: Sodium bisulfite is used as a reducing agent in various chemical processes. It can react with certain compounds, such as aldehydes and ketones, to convert them into their corresponding alcohols.
Water Treatment: Sodium bisulfite is used in water treatment to remove chlorine and prevent oxidation. It acts as a chelating agent, binding to metal ions and preventing their precipitation, which can cause scale formation and other water quality issues.
Storage
Conditions: Store in a cool and dry place.

|

|

|

|

|

|
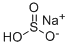
|

|
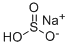
|

|

|
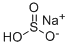
|

|
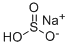
|
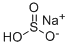
|
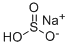
|
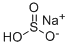
|
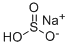
|
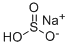
|
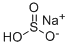
|