
|
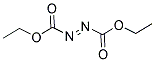
Diethyl azodicarboxylate, with the chemical formula C8H14N2O4, has the CAS number 1972-28-7. It appears as a colorless to pale yellow liquid with a pungent odor. The basic structure of Diethyl azodicarboxylate consists of two ethyl groups attached to a central nitrogen atom, which is in turn bonded to two carboxylate groups. This compound is sparingly soluble in water. Diethyl azodicarboxylate may be harmful if swallowed, inhaled, or absorbed through the skin. It can cause irritation to the respiratory system, skin, and eyes. In case of contact, immediate medical attention is advised. Diethyl azodicarboxylate is a flammable liquid and should be kept away from heat, sparks, and open flames. It should also be stored in a cool, well-ventilated area away from oxidizing agents.
Applicable Fields
Organic Synthesis: Diethyl azodicarboxylate is commonly used as a reagent in organic synthesis. Its purpose in this field involves its ability to act as a mild and selective oxidizing agent. The mechanism of action in organic synthesis involves the transfer of nitrogen atoms to organic compounds, facilitating various reactions such as the synthesis of aldehydes, ketones, and esters.
Pharmaceuticals: Diethyl azodicarboxylate has applications in the pharmaceutical industry. Its purpose in this field involves its ability to participate in the synthesis of various pharmaceutical compounds. The mechanism of action in pharmaceuticals involves its role as a versatile reagent for the preparation of amides, hydrazones, and other functional groups.
Storage
Conditions: Store in a cool, dry place.

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|