 |
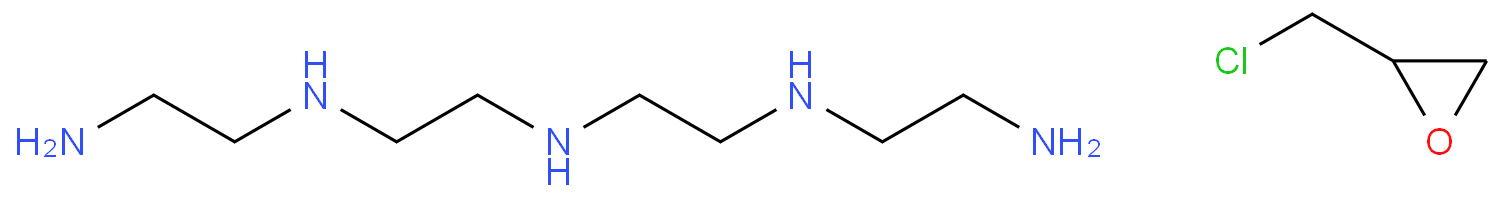
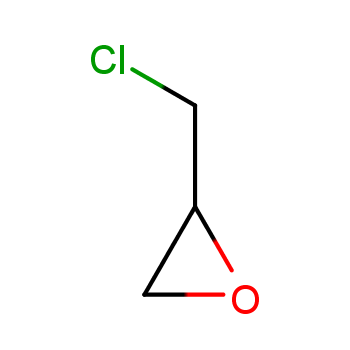
Epichlorohydrin (ECH) is an important organic chemical raw material and a significant intermediate in the petrochemical industry. It is mainly used for the production of epoxy resins, glycerol, chlorinated polyols, and other derivatives. It can also be used as a solvent, plasticizer, flame retardant, and surfactant. The ECH industry in China has experienced significant market changes in recent years, driven by the rapid growth in demand for epoxy resins due to their increasing applications.
Looking back at the world history of ECH, it can be said that it was first discovered by French chemist Marcellin Berthelot. In 1854, Berthelot treated crude glycerol with hydrochloric acid and then hydrolyzed it with alkali, accidentally obtaining ECH. Several years later, Reboul proposed that this substance could be obtained by the hydrolysis reaction of dichlorohydrin with caustic soda. It was not until 1948, ninety years later, that Shell, an American company, built the world's first production facility for glycidyl ether using the high-temperature chlorination method, with ECH as an intermediate product, marking the beginning of large-scale industrial production.
Around the 1960s, to meet the development needs of epoxy resin production, ECH began to be produced mainly using chloropropene as the raw material. The industrial production methods of ECH mainly include the high-temperature chlorination method and the acrylate ester method. The former, as mentioned above, was the main production process worldwide until 2010, initially developed and produced by Shell. The latter was successfully developed by the Soviet Academy of Sciences and Showa Denko of Japan in the 1980s.
Currently, there are three main industrial production methods for ECH worldwide: the high-temperature chlorination method, the acrylate ester method, and the glycerol method. Among them, the high-temperature chlorination method is the most important production method domestically and internationally, while the glycerol method is expected to become the future direction of ECH technology due to its economic and environmental advantages.
1. Classic High-Temperature Chlorination Method
Main raw materials: propylene, chlorine, and lime milk
Main process: high-temperature chlorination of propylene - chlorination of allyl chloride to hypochlorous acid - saponification of dichlorohydrin - product refining

2. Acrylate Ester Method
Technical sources: Soviet Academy of Sciences, Showa Denko (Japan)
Main raw materials: propylene, oxygen, acetic acid, chlorine, lime
Main process: oxidation of propylene - esterification to form acrylate ester - hydrolysis of acrylate ester to form allyl alcohol - chlorination of allyl alcohol to form dichlorohydrin - saponification of dichlorohydrin

3. Glycerol Method
Main raw materials: glycerol, hydrogen chloride, lime milk, catalyst
Main process: reaction of glycerol with hydrogen chloride to obtain high-concentration intermediate dichlorohydrin - saponification of dichlorohydrin - product refining

4. Direct Oxidation of Allyl Chloride Method
This method originated in the 1980s when Interox Chemicals (UK) successfully synthesized Epichlorohydrin by using peroxyacid to react with allyl chloride at 100-110°C. Currently, the direct oxidation of allyl chloride to prepare ECH generally uses hydrogen peroxide, peroxyacid, alkyl hydroperoxide, molecular oxygen, etc., as oxygen sources. Among them, hydrogen peroxide has achieved significant breakthroughs by Sinopec, Dalian Institute of Chemical Physics, Jiangsu Yangnong, and other units. It is expected that multiple sets of equipment will be built in the next few years.
5. Other Methods
In addition to the above methods, there are the propylene aldehyde method developed by DOW and the acetone method developed by Asahi and Mitsubishi in Japan.
The propylene aldehyde method includes processes such as propylene oxidation to synthesize propylene aldehyde, chlorination of propylene aldehyde to synthesize 2,3-dichloropropionaldehyde, hydrogenation of dichloropropionaldehyde to synthesize dichlorohydrin, and saponification of dichlorohydrin. The selectivity of propylene aldehyde to 2,3-dichloropropionaldehyde is 75.5%, and the selectivity of hydrogenation of dichloropropionaldehyde to dichlorohydrin is 95%. This method reduces the consumption of chlorine and the discharge of organic chloride wastewater but has been difficult to industrialize due to high energy consumption.
The acetone method developed by Asahi and Mitsubishi includes processes such as chlorination of acetone to produce 1,3-dichloropropanone, hydrogenation of dichloropropanone to produce 1,3-dichloropropanol, and saponification of dichloropropanol. However, industrialization has not been achieved for this method as well.
 |
 |
 |
 |
 |
 |
 |