 |
Rhodium(II) acetate dimer is a green to dark green solid powder at room temperature and pressure, slightly hygroscopic, insoluble in water but soluble in common organic solvents including ethyl acetate, dichloromethane, etc. It is a rhodium acetate salt with the metal rhodium in the +2 oxidation state, exhibiting high catalytic activity, mainly used as a transition metal catalyst for catalyzing reactions such as cyclopropanation of olefins.
Rhodium(II) acetate dimer has good chemical stability and is generally not prone to deterioration, stable in air but hygroscopic. The substance can be recrystallized from water or ethanol to obtain solvates, heating these solvates to 120 ℃ will remove the solvent and yield Rhodium(II) acetate dimer. It is worth noting that this rhodium complex is stable below 240 ℃ but decomposes at higher temperatures. X-ray crystallography confirms the compound's dimeric ion structure.
One of the most important reactions of organic diazo compounds is the formation of tricyclic products with olefins and alkynes. If one is not willing to delve deep into catalyst screening, the most convenient method is to use Rhodium(II) acetate dimer as the catalyst, which generally yields satisfactory yields.
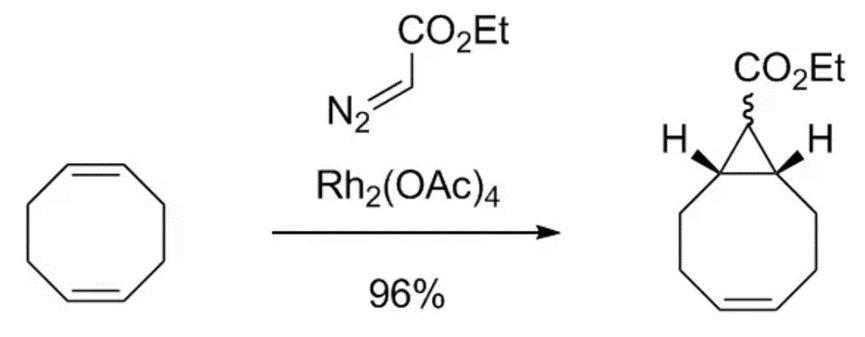
Figure 1 Rhodium(II) acetate dimer catalyzed cyclopropanation reaction
In 2018, the research group of Joseph M. Fox reported the cyclopropanation reaction of 1,5-cyclooctadiene catalyzed by Rhodium(II) acetate dimer, which can be scaled up for preparation.
Rhodium(II) acetate dimer is mainly used as a rhodium catalyst in organometallic chemistry, commonly used as a homogeneous catalyst for cyclopropanation reactions of olefins and allyl ethers. Its primary use in organic chemistry seems closely linked to reactions of organic diazo compounds, where Rhodium(II) acetate dimer is a convenient and widely applicable catalyst. The intramolecular reactions of diazo compounds with olefins are particularly significant, leading to fused ring products. Since this rhodium catalyst readily coordinates with chiral ligands to form chiral complexes, achieving stereo-selective reactions to obtain chiral cyclic products.
[1] Joseph M. Fox, J. Org. Chem. 2018, 83, 7500–7503.
 |