 |
In this article, we delve into the methods for preparing Acetohydroxamic acid and determining its content, providing valuable insights into the production and quality assessment of this important chemical compound with diverse applications.
1. Introduction:
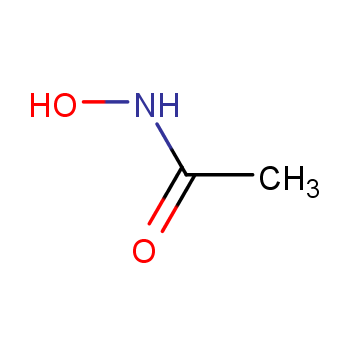
Acetohydroxamic acid (Acetohydroxamic acid, AHA) is one of the hydroxamic acid compounds, which was developed in the 1970s to treat urinary stones. and urinary tract infection drugs, and has been applied to hepatic coma caused by hyperammonemia. Acetohydroxamic acid reacts with urease The nickel atom chelates to inactivate urease, prevent urea from being decomposed, restore the normal pH of urine, improve the physiological environment of the urethra, prevent the formation of stones and inhibit their growth. Since AHA has the —CONHOH functional group, it is not only weakly acidic and has certain antibacterial activity, but is also a good organic ligand, which can not only gradually dissolve the stones , shrink or even disappear, and can also be used to prevent and treat urinary tract intubation stones and urinary tract stones, making it widely used in medicine, agriculture and metallurgical industries.
2. Preparation process:
2.1 In the existing preparation process, the common synthesis method of Acetohydroxamic acid is to react ethyl acetate or methyl acetate with hydroxylamine in a solvent system.
For example, (1) Acetohydroxamic acid is prepared by reacting hydroxylamine hydrochloride and ethyl acetate in an alkaline alcohol solution; (2) Acetohydroxamic acid is prepared by reacting hydroxylamine hydrochloride and methyl acetate in an alkaline alcohol solution; (3) Acetohydroxamic acid is prepared by using hydroxylamine sulfate Acetohydroxamic acid is produced by reacting with ethyl acetate under the catalysis of ethanol solution of sodium ethoxide; (4) Acetohydroxamic acid is synthesized using nitroethane method; (5) Hydroxylamine hydrochloride and acetamide are used under the action of catalyst 4-dimethylaminopyridine Acetohydroxamic acid is generated.
2.2 There is research on improving the traditional process by reacting ethyl acetate and hydroxylamine hydrochloride in a system using methanol as the solvent to produce Acetohydroxamic acid.
2.2.1 Synthesis principle
Taking advantage of the strongest activity of hydroxylamine under alkaline conditions, AHA is synthesized by reacting hydroxylamine hydrochloride and ethyl acetate in an alkaline solvent system. The main reaction process is as follows.
CH3COOC2H5+NH 2OH→CH 3CONHOH+ C 2H 5OH
2.2.2 Experimental steps
Add 21.0 g (0.30 mol) hydroxylamine hydrochloride and 120 mL methanol, heat and stir to completely dissolve hydroxylamine hydrochloride. Then add 16.0 g (0.40 mol) sodium hydroxide solid, stir and cool to room temperature in an ice bath, slowly add 36 mL of ethyl acetate dropwise, and react at room temperature until the reaction is complete. Then, while cooling in a water bath, slowly add concentrated hydrochloric acid dropwise to adjust the pH to 6.0~6.5, and continue stirring for 30 minutes. Filter, and wash the filter cake with an appropriate amount of methanol. The solvent is recovered from the filtrate under normal pressure to obtain crude product Product (light yellow substance). At 75°C, use ethyl acetate (4 × 100 mL) to heat and reflux to extract the product, filter while hot, and filter. The liquid was cooled and crystallized at 0~-5°C, filtered, and dried to obtain 19.6 g Acetohydroxamic acid, with a yield of 87.2%, mp: 88.2~90°C, and a purity of 98.3% when tested according to the United States Pharmacopoeia method.
3. Content determination:
The 1994 Ministry of Health standard uses the potassium permanganate method to determine AHA content, but this method is cumbersome to operate. Reported by Huang Shuling, Jun Yinghua and others The UV spectrophotometry method is relatively simple. In this method, ferric chloride solution needs to be added to react with AHA under acidic conditions to produce a complex and then colorimetric. However, the complex is unstable and cannot be maintained. The time is not long, which is not conducive to the detection of AHA content. Some researchers have separately explored fixed pH titration and high-phase liquid chromatography to detect AHA content, avoiding the use of complexes and combining 2 span>Compare the effectiveness of the two methods and obtain satisfactory results
References:
[1]. Liu Qiang, Cui Jianlan and Wu Yanbin, Comparison of the effectiveness of fixed pH titration and high performance liquid chromatography in determining the content of Acetohydroxamic acid. Modern Chemicals, 2013. 33(12): Pages 133-135.
[2]. Peng Junying et al., Synthesis study of Acetohydroxamic acid. Fine Chemical Intermediates, 2018. 48(02): Pages 47-49.
 |