 |
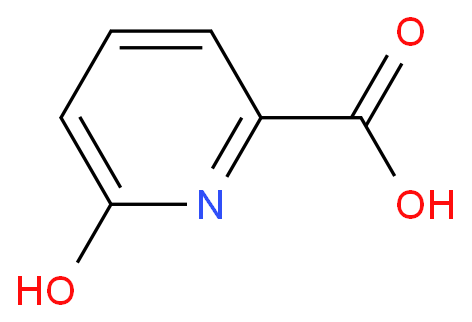
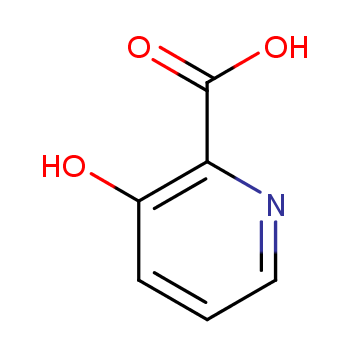
This article will explore the method of synthesizing 3- hydroxyl group -2- pyridin acid, so that readers will be able to better understand the preparation process of the compound.
Background: Pyridine carboxylic acid ligand is a classic two -tooth ligand containing nitrogen atoms and carboxyl oxygen atoms. Different pH has different degree of desertification of carboxyl genes. Its oxygen atom has a variety of coordination modes and has a variety of structures and unique properties.
3-hydroxyl group -2- pyridinyate derivatives are one of the common pyridine carboxylic acid ligands. At present, except 3- hydroxyl group -2- pyridine " (HO-PBA) , other 3- hydroxyl < SPAN> -2- Pyrazide methacology derivatives are expensive. HO-PBA is used as a ligand in metal organic mathematics. >] Cobalt (II) The mat and the matrix shows a good catalytic performance in the siliconized reaction of benzaldehyde and the reaction of benzylide and benzaldehyde. In addition, 3- hydroxyl group -2-
Synthetic:
1. Method 1:
Its commonly used synthetic method is to use
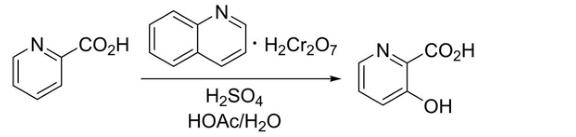
2. Method two:
This method contains oxidation reactions, cyanomized reactions and hydrolysis reactions. The oxidation reaction is based on the formula i3 ? Hydroxylpyamine and its derivatives are as substrates. Under the condition of oxidant in the hydrogen peroxide solution, 60 ° C in the nitrogen environment under nitrogen environment is under nitrogen environment. React to obtain the oxidation product shown in the structured II . Cyanidized reaction, based on compounds
{-127051568}
Called 1.000g 3- hydroxylpyamine (10.5 mmol) dissolved in 10.0ml ice acetic acid, add 1.315g < /SPAN> Hydrogen peroxide solution (30 % (w/w) , 11.6 mmol) , nitrogen protection 60 ℃ React 12 Stir in an hour. The thin -layer chromatography monitoring the reaction of the raw material completely, and the reaction solution is directly dried, and the petroleum ether and a small amount of ethyl acetate are filtered. It is dry and obtains the 1.210g oxidation product. Solk the previous step of oxidation products as raw materials in 12.1ml dichloromethane, add 1.350g trigenocyanane (13.6mmol) Add 1.195g dihyunamide (11.1 mmol) under zero -degree ice bath. The thin -layer chromatography monitoring the reaction raw material reaction is completely, adding the reaction raw solution to the potassium carbonate aquatic solution, and the pH value to 8 ; use 5.0ml Dichloromethane extraction twice, and then add dilute hydrochloric acid or dilute sulfuric acid to the water phase pH to 6 , precipitate a large amount of solid products; filter, and use 5.0 ML Petroleum ether was washed and dried in vacuum the filter cake to obtain 0.968g cyanomized products. Solk the previously cyanocytic product as the ingredients in the 10.0ml ethanol, and then add the sodium hydroxide water solution of 4.033g (w/w) , 20.2mmol) , and then return 12 hours, thin layer chromatography monitoring reaction raw materials react. Remove ethanol directly, and then add 5.0ml ethyl acetate to extract, and then adjust the water phase with dilute hydrochloric acid to 4 to precipitate solids , Then filter, wash cake water, dry the product 0.968g , target product 3- hydroxyl -2- pyridinyl acid isolated produce The rate is 66 %.
References:
[1] Liu Yuling Zhuo Xin Wei Qiang and other > Hydroxyl -2- Pyridine methylic acid is the synthesis and crystal structure of the nickel parts polymer of the ligand [j]. Chemical World , 2018,59 (6 ): 360-364. Doi: 10.19500/J.CNKI.0367-6358.20170609.
[2] Hainan Fan Sheng Biotechnology Co., Ltd. . A synthetic 3- hydroxyl -2- pyridine metic acid and its derivatives < span>: CN202011006099.8 [P]. 2020-12-22.
 |
 |
 |
 |
 |
 |
 |