In recent years, indoor air pollution has attracted attention from the whole society. Volatile organic compounds (VOCs) are important components of indoor pollution, with Hexanal being a common aldehyde ketone substance indoors. It has a high concentration indoors and its low odor threshold makes it easily perceptible, yet research on the catalytic removal of Hexanal is limited. Among common transition metal oxide catalysts, manganese oxide is widely studied due to its easy availability, high catalytic activity, and low toxicity. γ-MnOOH, a common hydroxyl manganese oxide, is often used as a precursor for synthesizing other manganese oxides and has wide applications in supercapacitors, ion batteries, and catalysis. This article will introduce its application in catalyzing the degradation of Hexanal.
Experimental Method
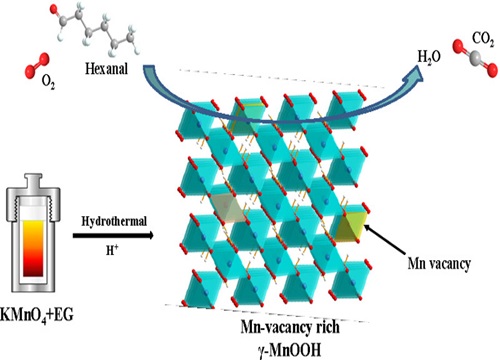
γ-MnOOH catalysts were prepared through two hydrothermal reaction systems: manganese acetate-peroxide (Mn(AC)2+H2O2) and potassium permanganate-ethylene glycol (KMnO4+EG). Adding a certain amount of sulfuric acid in the potassium permanganate-ethylene glycol system significantly increased the manganese defect content in the prepared γ-MnOOH catalyst material, enhancing the migration and conversion ability of lattice oxygen and facilitating the activation of oxygen to produce superoxide radicals (?O2-). This greatly improved the catalytic performance of the catalyst on Hexanal. Qualitative analysis of gas-phase and surface intermediate species during the catalytic oxidation of hexanal suggested a stepwise oxidative decarboxylation and degradation process.
Experimental Conclusion
1. γ-MnOOH prepared from the reaction of peroxide and manganese acetate had good crystallinity, small surface area, and few surface defects, resulting in weak catalytic oxidation activity towards Hexanal. γ-MnOOH prepared from the reaction of potassium permanganate and ethylene glycol had a relatively higher surface area, easier formation of surface defects, and stronger catalytic performance towards Hexanal.
2. Adding a certain amount of sulfuric acid in the potassium permanganate and ethylene glycol reaction system could produce γ-MnOOH catalyst material with higher manganese defect content, enhanced migration and conversion ability of lattice oxygen, and easier activation of oxygen to produce superoxide radicals (?O2-), significantly improving the catalytic performance of the catalyst on Hexanal.
3. Qualitative analysis of gas-phase products and surface intermediate species in the catalytic system revealed that when Hexanal was not completely converted, only n-hexanal and n-butanal were detected in the gas phase products. Carboxylic acid substances were also detected on the catalyst surface during in-situ infrared testing, suggesting that the catalytic oxidation of Hexanal in the gas phase is a stepwise degradation process.
References
[1] Low-temperature catalytic degradation of the odorous pollutant hexanal by γ-MnOOH: The effect of Mn vacancies. doi: 10.1016/S1872-2067(19)63415-7


![3-Boc-3-azabicyclo[3.1.0]hexane-1-Methanol](https://structimg.guidechem.com/11/59/3077338.png)


![(1R,2S,5S)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate (2S,3S)-2,3-bis(4-Methylbenzoyloxy)succinate](https://structimg.guidechem.com/11/47/1990906.png)


