
|
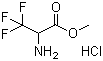
Methyl 2-amino-3, 3, 3-trifluoropropanoate hydrochloride (820 mg, 4.24 mmol) was added to 4- (dibenzylamino)cyclohexanone (Intermediate 63A) (1 .24 g, 4.24 mmol) in 1 , 2- dichloroethane (21 mL) at room temperature and stirred for 5 minutes, followed by 4A molecular sieves (10 g). After 2 h, sodium bicarbonate (356 mg, 4.24 mmol) and sodium triacetoxyborohydride (0.898 g, 4.24 mmol) were added, and the reaction mixture was stirred overthe weekend. The reaction mixture was filtered, diluted with EtOAc, washed with saturated aqueous NaHCCb and the aqueous layer was extracted with EtOAc. The combined organics were washed with brine, dried over magnesium sulfate, filtered, and concentrated. The residue was purified by silica gel chromatography, eluting with 2:3 EtOAc:hexanes to give the title compound (730 mg, 40% yield) as a mixture of cis and trans isomers. 1H NMR (400 MHz, CD3SOCD3) delta 0.98-1 .20 (m, 2 H), 1 .31 -2.01 (m, 6 H), 2.37-2.47 (m, 1 H), 2.48-2.58 (m, 1 H), 2.75 (br s, 1 H), 3.59 (s, 2 H), 3.64 (s, 2 H), 3.74-3.84 (m, 1 H), 3.84 (s, 3 H), 7.19-7.25 (m, 2 H), 7.27-7.33 (m, 4 H), 7.34-7.42 (m, 4 H); LC-MS (LC-ES) M+H = 435.Example 28.2-Cyclopropyl-5H-pyrrolo[2, 3b]pyrazine-7-carboxylic acid (2-hydroxy-2-methyl-l- trifluoromethyl-propyl)-amideStep 1Methyl 3, 3, 3-trifluoroalaninate hydrochloride (1.0 g, 5.16 mmol) was dissolved in 26 ml of dichloromethane. Triethylamine (0.72 ml, 5.16 mmol) was added and the reaction was cooled in an ice bath. Di-ieri-butyldicarbonate (2.2 g, 10.3 mmol) was added slowly and the reaction was stirred for 18 h. Ethyl acetate and ammonium chloride solution were added, the layers were separated and the aqueous layer was extracted once more with ethyl acetate. The combined organic layers were washed with sodium chloride solution and dried over sodium sulfate. After evaporation the residue was purified by silica gel chromatography to give 2- tert-butoxycarbonylamino-3, 3, 3-trifluoro-propionic acid methyl ester.
|

|

|

|

|

|

|

|

|

|