ALUMINUM CARBIDE is a yellow powder or crystals. Used to make other chemicals.
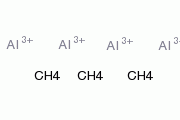
Aluminum Carbide, with the chemical formula Al4C3 and CAS number 1299-86-1, is a compound consisting of four aluminum atoms and three carbon atoms. It appears as a dark gray powder with no distinct odor. Aluminum Carbide is insoluble in water and is known for its stability under normal conditions. Safety information regarding Aluminum Carbide suggests that it may pose respiratory and skin irritation risks. Additionally, exposure to moisture or water can lead to the release of flammable gases. Adequate protective measures should be taken during handling to minimize potential health hazards.
Applicable Fields
Industrial Applications: Aluminum Carbide finds use in industrial processes, serving as a source of acetylene gas when it reacts with water. The mechanism of action involves the hydrolysis of Aluminum Carbide, producing acetylene gas, which has applications in various industries, including welding and metal cutting.
Chemical Synthesis: This compound is utilized in chemical synthesis as a precursor to other chemicals. The mechanism of action involves its participation in reactions leading to the formation of different chemical products, expanding its role in various synthetic processes.
Storage
ConditionsStore in a dry, cool place, away from moisture sources.